

All other common household or classroom materials can be saved or disposed of in the usual manner. Save the bottles and sand for future use.
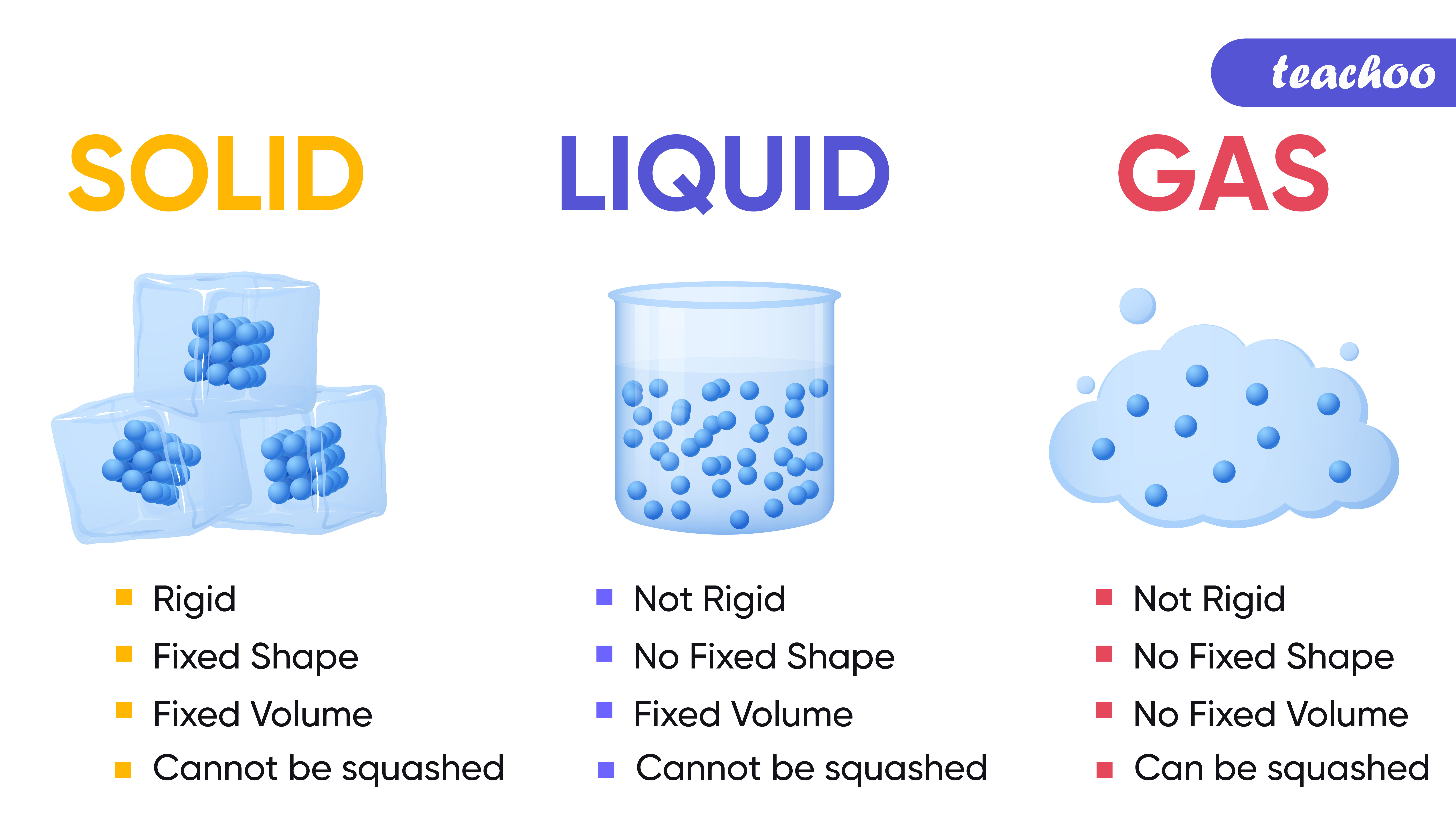
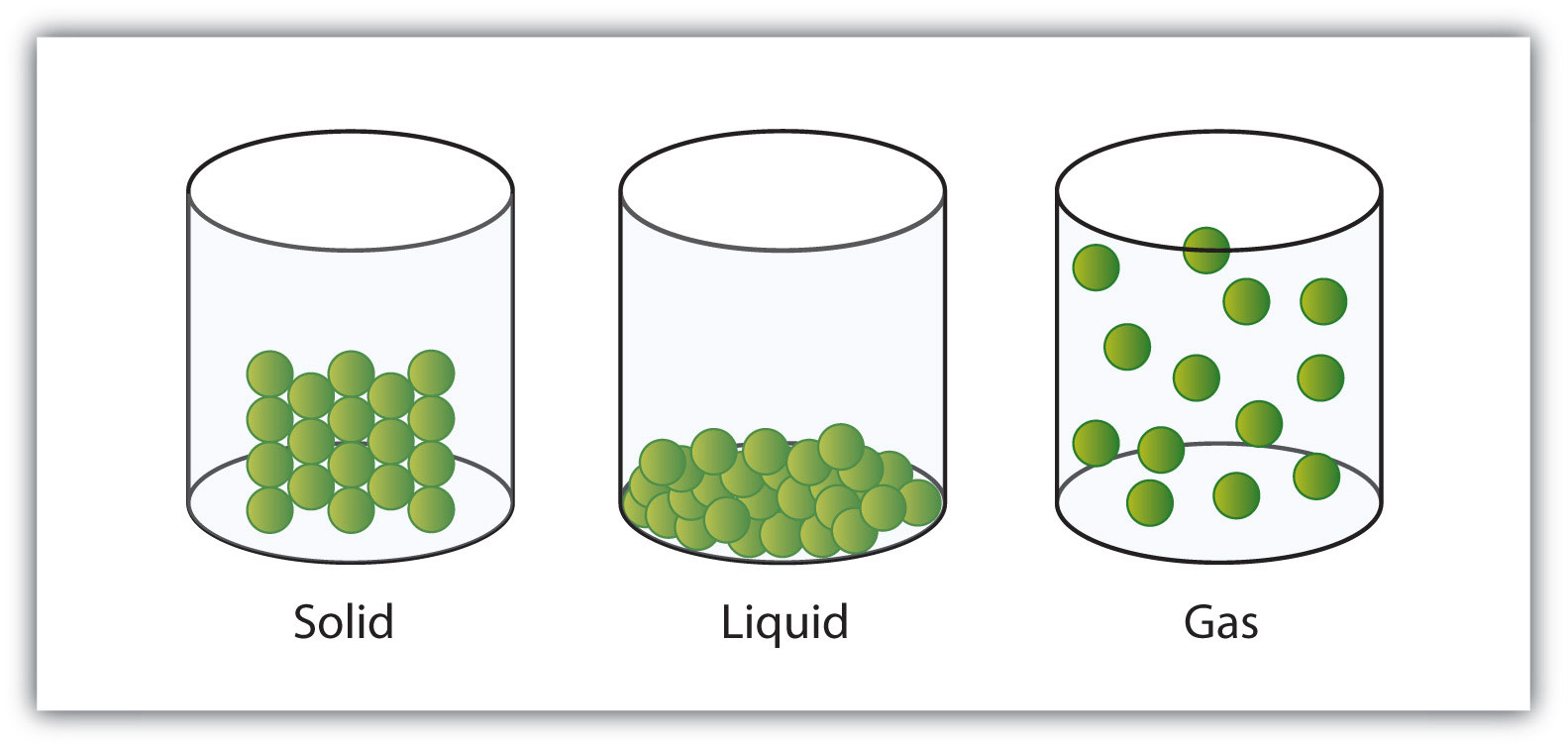
Remind students to wash their hands after completing the activities. Make sure you and your students wear properly fitting safety glasses or goggles. The activity sheet will serve as the Evaluate component of the 5-E lesson plan. Print the student activity sheet and distribute one per student when specified in the activity. Finally, students make an argument that even though a mound of shaving cream keeps its shape, it is not a solid, and that even though sand takes the shape of its container, it is not a liquid.Students watch a short animation that illustrates the incredibly tiny size of atoms and molecules.Students also try to squeeze a bottle filled with water to develop a model of the particles of a liquid.Students squeeze a flexible plastic bottle with a balloon on top of the bottle to develop a model of the particles of a gas.Students observe a solid metal hammer and a nail and view a molecular model animation of the particles in a solid.Students are introduced to the idea that matter is made up of tiny particles called atoms and molecules.NGSS 5-PS1-1: Develop a model to describe that matter is made of particles too small to be seen.The particles don’t interact with one another but just hit and bounce off of each other when they collide. They are very far apart compared to the particles in a solid or liquid, and are constantly moving. In a gas, the particles have very little attraction to each other.The particles of a liquid are close together, always moving, and can slide past one another. In a liquid, the particles are attracted to each other but not as much as they are in a solid.

They are close together and vibrate in position but don’t move past one another. In a solid, the particles are very attracted to each other.Solids, liquids, and gases are made of tiny particles called atoms and molecules.Matter on Earth is in the form of solid, liquid, or gas.Finally, students will use their models of solids, liquids, and gases to explain their observations in the lesson. Students will use the model to describe the differences in attraction among the particles of a solid, liquid, and gas. Recreating this unusual state in other materials could have all kinds of applications.Students will develop a model to describe that matter is made up of tiny particles, too small to be seen. We have shown that this unusual but stable state is part solid and part liquid. Potassium is one of the simplest metals we know, yet if you squeeze it, it forms very complicated structures. The work was carried out in collaboration with scientists from Xi’an Jiantong University in China. The study, published in the journal Proceedings of the National Academy of Sciences, was supported by the European Research Council and the Engineering and Physical Sciences Research Council. Simulating how up to 20,000 potassium atoms behave under extreme conditions revealed that the structures formed represent the new, stable state of matter.Īpplying pressure to the atoms leads to the formation of two interlinked solid lattice structures, the team says.Ĭhemical interactions between atoms in one lattice are strong, meaning they stay in a solid form when the structure is heated, while the other atoms melt into a liquid state. High performance computingĪ team led by scientists from the University of Edinburgh used powerful computer simulations to study the existence of the state – known as the chain-melted state.
Until now, it was unclear if the unusual structures represented a distinct state of matter, or existed as transition stages between two distinct states. Under the right conditions, over half a dozen elements – including sodium and bismuth – are thought to be capable of existing in the newly discovered state, researchers say. However, the structure also contains a second set of potassium atoms that are in a fluid arrangement. Novel structureĪpplying high pressures and temperatures to potassium – a simple metal – creates a state in which most of the element’s atoms form a solid lattice structure, the findings show. Researchers have found, however, that some elements can, when subjected to extreme conditions, take on the properties of both solid and liquid states. Until now, the atoms in physical material were understood to exist typically in one of three states – solid, liquid or gas. Scientists have discovered a new state of physical matter in which atoms can exist as both solid and liquid simultaneously.


 0 kommentar(er)
0 kommentar(er)
